Cerium is the undisputed ‘big brother’ in the large family of rare earth elements. Firstly, the total abundance of rare earths in the crust is 238ppm, with cerium at 68ppm, accounting for 28% of the total rare earth composition and ranking first; Secondly, cerium is the second rare earth element discovered nine years after the discovery of yttrium (1794). Its application is very extensive, and “cerium” is unstoppable
The Discovery of Cerium Element

Carl Auer von Welsbach
Cerium was discovered and named in 1803 by German Kloppers, Swedish chemist J ö ns Jakob Berzelius, and Swedish mineralogist Wilhelm Hisinger. It is called ceria, and its ore is called cerite, in memory of Ceres, an asteroid discovered in 1801. In fact, this type of cerium silicate is a hydrated salt containing 66% to 70% cerium, while the rest are compounds of calcium, iron, and yttrium.
The first use of cerium was a gas fireplace invented by Austrian chemist Carl Auer von Welsbach. In 1885, he attempted a mixture of magnesium, lanthanum, and yttrium oxide, but these mixtures emitted a green light without success.
In 1891, he found that pure thorium oxide produced a better light, although it was blue, and mixed with Cerium(IV) oxide to produce a bright white light. In addition, Cerium(IV) oxide can also be used as a catalyst for thorium oxide combustion
Cerium metal

★ Cerium is a ductile and soft silver white metal with active properties. When exposed to air, it will be oxidized, forming a rust like peeling oxide layer. When heated, it burns and reacts quickly with water. A centimeter sized cerium metal sample completely corrodes within about a year. Avoid contact with air, strong oxidants, strong acids, and halogens.
★ Cerium mainly exists in monazite and bastnaesite, as well as in fission products of uranium, thorium, and plutonium. Harmful to the environment, special attention should be paid to pollution of water bodies.
★ Cerium is the 26th most abundant element, accounting for 68ppm of the Earth’s crust, second only to copper (68ppm). Cerium is more abundant than ordinary metals such as lead (13pm) and tin (2.1ppm).
Cerium Electron configuration
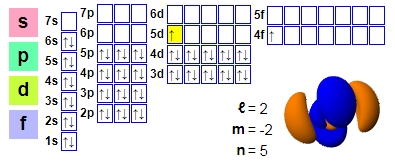
Electronic arrangements:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p66s2 4f1 5d1
★ Cerium is located after lanthanum and has 4f electrons starting from cerium, making it easy to participate in chemical reactions. However, the 5d orbital of cerium is occupied, and this effect is not strong enough in cerium.
★ Most Lanthanide can only use three electrons as Valence electron, with the exception of cerium, which has a variable electronic structure. The energy of 4f electrons is almost the same as that of the external 5d and 6s electrons delocalized in the metal state, and only a small amount of energy is needed to change the relative occupation of these electronic energy levels, resulting in the double valence of+3 and+4. The normal state is+3 valence, showing+4 valence in anaerobic water.
Application of cerium

★ Can be used as an alloy additive and for the production of cerium salts, etc.
★ It can be used as glass additive to absorb ultraviolet and infrared rays, and is widely used in Car glass.
★ Can be used as an excellent environmental protection material, and currently the most representative is the automotive exhaust purification catalyst, which effectively prevents a large amount of automotive exhaust gas from being discharged into the air.
★ Light rare earth elements mainly composed of cerium as plant growth regulators can improve crop quality, increase yield, and enhance crop stress resistance.
★ Cerium sulfide can replace metals such as lead and cadmium that are harmful to the environment and human beings in pigments, can color plastics, and can also be used in coatings and ink industries.
★ Cerium(IV) oxide can be used as polishing compound, for example, in Chemical-mechanical polishing (CMP).
★ Cerium can also be used as hydrogen storage materials, thermoelectric materials, cerium tungsten electrodes, Ceramic capacitor, piezoelectric ceramics, cerium silicon carbide abrasives, fuel cell raw materials, gasoline catalysts, permanent magnetic materials, medical materials, various alloy steels and non-ferrous metals.
Post time: Jul-03-2023