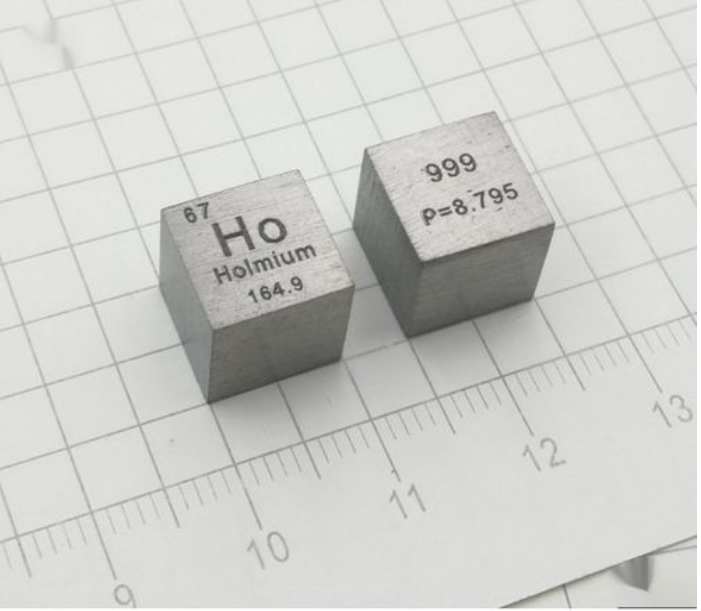
Holmium, atomic number 67, atomic weight 164.93032, element name derived from the birthplace of the discoverer.
The content of holmium in the crust is 0.000115%, and it exists together with other rare earth elements in monazite and rare earth minerals. The natural stable isotope is only holmium 165.
Holmium is stable in dry air and oxidizes quickly at high temperatures; Holmium oxide is known to have the strongest paramagnetic properties.
The compound of holmium can be used as an additive for new ferromagnetic materials; Holmium iodide is used to manufacture metal halide lamps – holmium lamps, and holmium lasers are also widely used in the medical field.
Discovering History
Discovered by: J.L. Soret, P.T. Cleve
Discovered from 1878 to 1879
Discovery process: discovered by J.L. Soret in 1878; Discovered by P.T. Cleve in 1879
After Mossander separated erbium earth and terbium earth from yttrium earth in 1842, many chemists used spectral analysis to identify and determine that they were not pure oxides of an element, which encouraged chemists to continue separating them. After separating ytterbium oxide and scandium oxide from oxidized bait, Cliff separated two new elemental oxides in 1879. One of them is named Holmium to commemorate Cliff’s birthplace, the ancient Latin name Holmia in Stockholm, Sweden, with the elemental symbol Ho. In 1886, another element was separated from holmium by Bouvabadrand, but the name of holmium was retained. With the discovery of holmium and other rare earth elements, another stage of the third discovery of rare earth elements has been completed
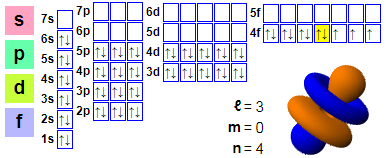
Electronic layout:
Electronic layout:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f11
It is a metal that, like dysprosium, can absorb neutrons produced by nuclear fission.
In a nuclear reactor, on the one hand, continuous combustion is carried out, and on the other hand, the speed of the chain reaction is controlled.
Element Description: The first ionization energy is 6.02 electron volts. Has a metallic luster. It can slowly react with water and dissolve in dilute acids. Salt is yellow. The oxide Ho2O2 is light green. Dissolve in mineral acids to produce trivalent ion yellow salts.
Element source: prepared by reducing holmium fluoride HoF3 · 2H2O with calcium.
Metal
Holmium is a silver white metal with soft texture and ductility; Melting point 1474 ° C, boiling point 2695 ° C, density 8.7947 g/cm holmium meter ³ 。
Holmium is stable in dry air and oxidizes quickly at high temperatures; Holmium oxide is known to have the strongest paramagnetic properties.
Obtaining compounds that can be used as additives for new ferromagnetic materials; Holmium iodide used in the manufacturing of metal halide lamps – holmium lamps
Application
(1) As an additive for metal halide lamps, metal halide lamps are a type of gas discharge lamp developed on the basis of high-pressure mercury lamps, characterized by filling the bulb with various rare earth halides. At present, the main use is rare earth iodide, which emits different spectral colors during gas discharge. The working substance used in holmium lamps is holmium iodide, which can achieve a high concentration of metal atoms in the arc zone, greatly improving radiation efficiency.
(2) Holmium can be used as an additive for yttrium iron or yttrium aluminum garnet.
(3) Ho: YAG doped yttrium aluminum garnet can emit 2 μ M laser, human tissue on 2 μ The absorption rate of m laser is high, almost three orders of magnitude higher than that of Hd: YAG. So when using Ho: YAG laser for medical surgery, not only can the surgical efficiency and accuracy be improved, but also the thermal damage area can be reduced to a smaller size. The free beam generated by holmium crystals can eliminate fat without generating excessive heat, thereby reducing thermal damage to healthy tissues. It is reported that holmium laser treatment for glaucoma in the United States can reduce the pain of patients undergoing surgery. China 2 μ The level of m laser crystals has reached international level, and efforts should be made to develop and produce this type of laser crystal.
(4) In the magnetostrictive alloy Terfenol D, a small amount of holmium can also be added to reduce the external field required for saturation magnetization of the alloy.
(5) The use of holmium doped fiber can make optical communication devices such as fiber lasers, fiber amplifiers, and fiber sensors, which will play a more important role in the rapid development of fiber optic communication today.
(6) Holmium laser lithotripsy technology: Medical holmium laser lithotripsy is suitable for hard kidney stones, ureteral stones, and bladder stones that cannot be broken by extracorporeal shock wave lithotripsy. When using medical holmium laser lithotripsy, the slender fiber of the medical holmium laser is used to directly reach the bladder, ureter, and kidney stones through the urethra and ureter through a cystoscope and ureteroscope. Then, urology experts manipulate the holmium laser to break the stones. The advantage of this holmium laser treatment method is that it can solve ureteral stones, bladder stones, and the vast majority of kidney stones. The disadvantage is that for some stones in the upper and lower renal calices, there may be a small amount of residual stones due to the inability of the holmium laser fiber entering from the ureter to reach the stone site.
Post time: Aug-16-2023