Praseodymium is the third most abundant lanthanide element in the periodic table of chemical elements, with an abundance of 9.5 ppm in the crust, only lower than cerium, yttrium, lanthanum, and scandium. It is the fifth most abundant element in rare earths. But just like his name, praseodymium is a simple and unadorned member of the rare earth family.
C.F. Auer Von Welsbach discovered praseodymium in 1885.
In 1751, Swedish mineralogist Axel Fredrik Cronstedt found a heavy mineral in the Bastn ä s mining area, which was later named cerite. Thirty years later, fifteen year old Vilhelm Hisinger from the family that owned the mine sent his samples to Carl Scheele, but he did not discover any new elements. In 1803, after Singer became a blacksmith, he returned to the mining area with J ö ns Jacob Berzelius and separated a new oxide, the dwarf planet Ceres, which they discovered two years ago. Ceria was separated independently by Martin Heinrich Klaproth in Germany.
Between 1839 and 1843, Swedish surgeon and chemist Carl Gustaf Mosander discovered that cerium oxide was a mixture of oxides. He separated two other oxides, which he called lanthana and didymia “didymia” (meaning “twins” in Greek). He partially decomposed the cerium nitrate sample by roasting it in the air, and then treated it with dilute nitric acid to obtain the oxide. The metals that form these oxides are therefore named lanthanum and praseodymium.
In 1885, C.F. Auer Von Welsbach, an Austrian who invented the thorium cerium vapor lamp gauze cover, successfully separated the “praseodymium neodymium”, the “conjoined twins”, from which green praseodymium salt and rose colored neodymium salt were separated and determined to be two new elements. One is named “Praseodymium”, which comes from the Greek word prason, meaning green compound because a solution of praseodymium salt water will present a bright green color; The other element is named “Neodymium“. The successful separation of the “conjoined twins” enabled them to display their talents independently.
Silver white metal, soft and ductile. Praseodymium has a hexagonal crystal structure at room temperature. The corrosion resistance in air is stronger than that of lanthanum, cerium, neodymium, and europium, but when exposed to air, a layer of fragile black oxide is produced, and a one centimeter sized praseodymium metal sample completely corrodes within about a year.
Like most rare earth elements, praseodymium is most likely to form a+3 oxidation state, which is its only stable state in aqueous solutions. Praseodymium exists in a+4 oxidation state in some known solid compounds, and under matrix separation conditions, it can reach a unique+5 oxidation state among lanthanide elements.
The aqueous praseodymium ion is chartreuse, and many industrial uses of praseodymium involve its ability to filter yellow light in light sources.
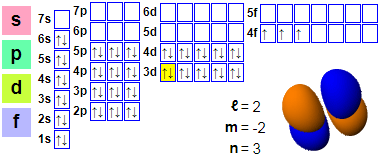
Praseodymium electronic layout
Electronic emissions:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p66s2 4f3
The 59 electrons of praseodymium are arranged as [Xe] 4f36s2. Theoretically, all five outer electrons can be used as valence electron, but the use of all five outer electrons requires extreme conditions. Generally, praseodymium only emits three or four electrons in its compounds. Praseodymium is the first lanthanide element with an electronic configuration that conforms to the Aufbau principle. Its 4f orbital has lower energy levels than the 5d orbital, which is not applicable to lanthanum and cerium, as the sudden contraction of the 4f orbital does not occur until after lanthanum and is not sufficient to avoid occupying the 5d shell in cerium. Nevertheless, solid praseodymium exhibits a [Xe] 4f25d16s2 configuration, where one electron in the 5d shell resembles all other trivalent lanthanide elements (except for europium and ytterbium, which are divalent in metallic states).
Like most lanthanide elements, praseodymium usually uses only three electrons as valence electron, and the remaining 4f electrons have strong binding effect: this is because the 4f orbit passes through the inert xenon core of the electron to reach the nucleus, followed by 5d and 6s, and increases with the increase of ionic charge. However, praseodymium can still continue to lose the fourth and even occasionally the fifth valence electron, because it appears very early in the lanthanide system, where the nuclear charge is still low enough, and the 4f subshell energy is high enough to allow the removal of more valence electron.
Praseodymium and all lanthanide elements (except lanthanum, ytterbium and lutetium, there are no unpaired 4f electrons) are paramagnetism at room temperature. Unlike other rare earth metals that exhibit antiferromagnetic or ferromagnetic ordering at low temperatures, praseodymium is paramagnetism at all temperatures above 1K
Application of Praseodymium
Praseodymium is mostly utilized in the form of mixed rare earths, such as as as a purifying and modifying agent for metal materials, chemical catalysts, agricultural rare earths, and so on. Praseodymium neodymium is the most similar and difficult to separate pair of rare earth elements, which is difficult to separate by chemical methods. Industrial production usually uses extraction and ion exchange methods. If they are used in pairs in the form of enriched praseodymium neodymium, their commonality can be fully utilized, and the price is also cheaper than single element products.
Praseodymium neodymium alloy (praseodymium neodymium metal) has become an independent product, which can be used as both a permanent magnet material and a modification additive for non-ferrous metal alloys. The activity, selectivity and stability of petroleum cracking catalyst can be improved by adding praseodymium neodymium concentrate into Y zeolite molecular sieve. As a plastic modification additive, adding praseodymium neodymium enrichment to polytetrafluoroethylene (PTFE) can significantly improve the wear resistance of PTFE.
Rare earth permanent magnet materials are the most popular field of rare earth applications today. Praseodymium alone is not outstanding as a permanent magnet material, but it is an excellent synergistic element that can improve magnetic properties. Adding an appropriate amount of praseodymium can effectively improve the performance of permanent magnet materials. It can also improve the antioxidant performance (air corrosion resistance) and mechanical properties of magnets, and has been widely used in various electronic devices and motors.
Praseodymium can also be used for grinding and polishing materials. As we all know, pure cerium based polishing powder is usually light yellow, which is a high-quality polishing material for optical glass, and has replaced the iron oxide red powder that has low polishing efficiency and pollutes the production environment. People have found that praseodymium has good polishing properties. Rare earth polishing powder containing praseodymium will appear reddish brown, also known as “red powder”, but this red color is not iron oxide red, but due to the presence of praseodymium oxide, the color of the rare earth polishing powder becomes darker. Praseodymium has also been used as a new grinding material to make corundum grinding wheels containing praseodymium. Compared with white alumina, efficiency and durability can be improved by more than 30% when grinding carbon structural steel, stainless steel, and high-temperature alloys. In order to reduce costs, praseodymium neodymium enriched materials were often used as raw materials in the past, hence the name praseodymium neodymium corundum grinding wheel.
Silicate crystals doped with praseodymium ions have been used to slow down light pulses to several hundred meters per second.
Adding praseodymium oxide to zirconium silicate will turn bright yellow and can be used as a ceramic pigment – praseodymium yellow. Praseodymium yellow (Zr02-Pr6Oll-Si02) is considered the best yellow ceramic pigment, which remains stable at up to 1000 ℃ and can be used for one-time or reburning processes.
Praseodymium is also used as a glass colorant, with rich colors and great potential market. Praseodymium green glass products with bright leek green and scallion green colors can be produced, which can be used to produce green filters and also for arts and crafts glass. Adding praseodymium oxide and cerium oxide to the glass can be used as goggles for welding. Praseodymium sulfide can also be used as a green plastic colorant.
Post time: May-29-2023