Europium, the symbol is Eu, and the Atomic number is 63. As a typical member of Lanthanide, europium usually has+3 valence, but oxygen+2 valence is also common. There are fewer compounds of europium with a valence state of+2. Compared with other heavy metals, europium has no significant biological effects and is relatively non-toxic. Most applications of europium use the phosphorescence effect of Europium compounds. Europium is one of the least abundant elements in the universe; There are only about 5 in the universe × 10-8% of the substance is europium.
Europium exists in monazite
The Discovery of Europium
The story begins at the end of the 19th century: at that time, excellent scientists began to systematically fill the remaining vacancies in Mendeleev’s periodic table by analyzing the Atomic emission spectrum. In today’s view, this job is not difficult, and an undergraduate student can complete it; But at that time, scientists only had instruments with low precision and samples that were difficult to purify. Therefore, in the whole history of the discovery of Lanthanide, all “quasi” discoverers kept making false claims and arguing with each other.
In 1885, Sir William Crookes discovered the first but not very clear signal of element 63: he observed a specific red spectral line (609 nm) in a samarium sample. Between 1892 and 1893, the discoverer of gallium, samarium, and dysprosium, Paul é mile LeCoq de Boisbaudran, confirmed this band and discovered another green band (535 nm).
Next, in 1896, Eug è ne Anatole Demar ç ay patiently separated samarium oxide and confirmed the discovery of a new rare earth element located between samarium and gadolinium. He successfully separated this element in 1901, marking the end of the discovery journey: “I hope to name this new element Europium, with the symbol Eu and the Atomic mass of about 151.”
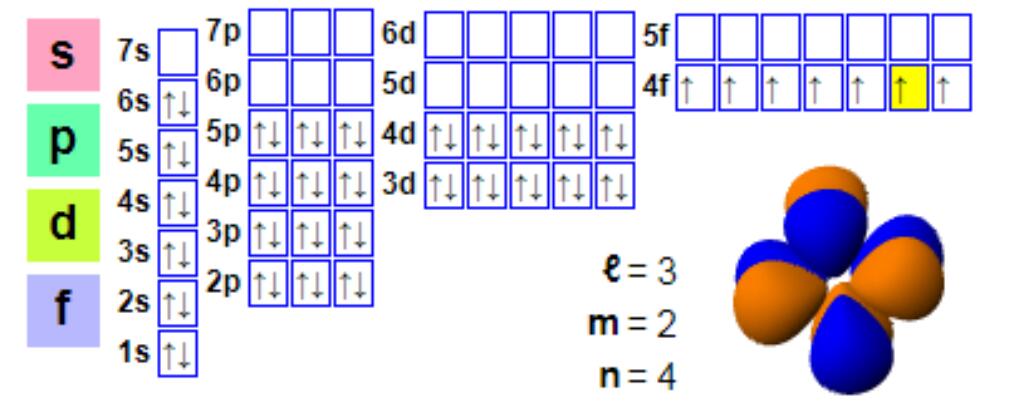
Electron configuration
Electron configuration:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p66s2 4f7
Although europium is usually trivalent, it is prone to forming divalent compounds. This phenomenon is different from the formation of+3 valence compounds by most Lanthanide. Divalent europium has an electronic configuration of 4f7, as the semi filled f shell provides more stability, and europium (II) and barium (II) are similar. Divalent europium is a mild reducing agent that oxidizes in air to form a compound of europium (III). Under anaerobic conditions, especially heating conditions, divalent europium is sufficiently stable and tends to be incorporated into calcium and other alkaline earth minerals. This ion exchange process is the basis of the “negative europium anomaly”, that is, compared with the abundance of Chondrite, many lanthanide minerals such as monazite have low europium content. Compared to monazite, bastnaesite often exhibits fewer negative europium anomalies, so bastnaesite is also the main source of europium.
Europium is an iron gray metal with a melting point of 822 ° C, a boiling point of 1597 ° C, and a density of 5.2434 g/cm ³; It is the least dense, softest, and most volatile element among rare earth elements. Europium is the most active metal among rare earth elements: at room temperature, it immediately loses its metallic luster in the air and is quickly oxidized into powder; React violently with cold water to generate hydrogen gas; Europium can react with boron, carbon, sulfur, phosphorus, hydrogen, nitrogen, etc.
Application of Europium
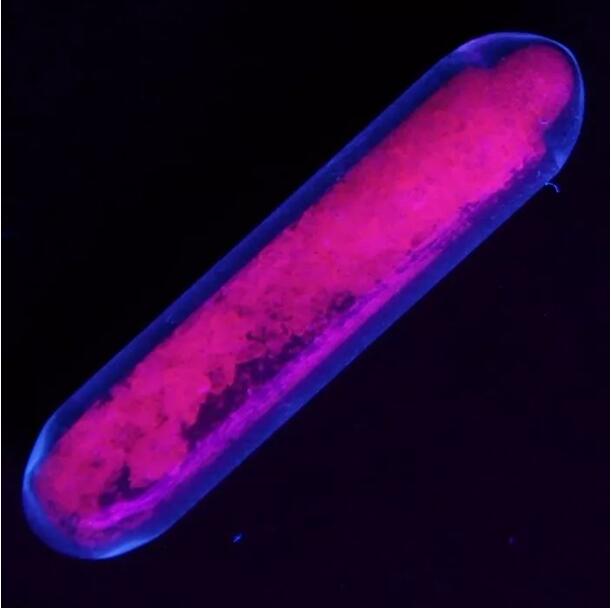
Europium sulfate emits red fluorescence under ultraviolet light
Georges Urbain, a young outstanding chemist, inherited the Spectroscopy instrument of Demar ç ay and found that a Yttrium(III) oxide sample doped with europium emitted very bright red light in 1906. This is the beginning of the long journey of europium phosphorescent materials – not only used to emit red light, but also blue light, because the emission spectrum of Eu2+falls within this range.
A phosphor composed of red Eu3+, green Tb3+, and blue Eu2+emitters, or a combination of them, can convert ultraviolet light into visible light. These materials play an important role in various instruments around the world: X-ray intensifying screens, cathode ray tubes or plasma screens, as well as recent energy-saving fluorescent lamps and light-emitting diodes.
The fluorescence effect of trivalent europium can also be sensitized by organic aromatic molecules, and such complexes can be applied in various situations that require high sensitivity, such as anti-counterfeiting inks and barcodes.
Since the 1980s, europium has been playing a leading role in highly sensitive biopharmaceutical analysis using time-resolved cold fluorescence method. In most hospitals and medical laboratories, such analysis has become routine. In the research of life science, including biological imaging, fluorescent biological probes made of europium and other Lanthanide are ubiquitous. Fortunately, one kilogram of europium is enough to support approximately one billion analyses – after the Chinese government recently restricted rare earth exports, industrialized countries panicked by rare earth element storage shortages do not have to worry about similar threats to such applications.
Europium oxide is used as Stimulated emission phosphor in new X-ray medical diagnosis system. Europium oxide can also be used to manufacture colored lenses and optoelectronic filters, for magnetic bubble storage devices, and in control materials, shielding materials, and structural materials of atomic reactors. Because its atoms can absorb more neutrons than any other element, it is commonly used as a material for absorbing neutrons in atomic reactors.
In today’s rapidly expanding world, the recently discovered application of europium may have profound impacts on agriculture. Scientists have found that plastics doped with divalent europium and univalent copper can efficiently convert the ultraviolet part of sunlight into visible light. This process is quite green (it is the Complementary colors of red). Using this type of plastic to build a greenhouse can enable plants to absorb more visible light and increase crop yields by approximately 10%.
Europium can also be applied to quantum memory chips, which can reliably store information for several days at a time. These can enable sensitive quantum data to be stored in a device similar to a hard disk and shipped across the country.
Post time: Jun-27-2023