1、 Definition of Nuclear Materials
In a broad sense, nuclear material is the general term for materials used exclusively in the nuclear industry and nuclear scientific research, including nuclear fuel and nuclear engineering materials, i.e. non nuclear fuel materials.
The commonly referred to nuclear materials mainly refer to materials used in various parts of the reactor, also known as reactor materials. Reactor materials include nuclear fuel that undergoes nuclear fission under neutron bombardment, cladding materials for nuclear fuel components, coolants, neutron moderators (moderators), control rod materials that strongly absorb neutrons, and reflective materials that prevent neutron leakage outside the reactor.
2、 Co associated relationship between rare earth resources and nuclear resources
Monazite, also called phosphocerite and phosphocerite, is a common accessory mineral in intermediate acid igneous rock and metamorphic rock. Monazite is one of the main minerals of rare earth metal ore, and also exists in some sedimentary rock. Brownish red, yellow, sometimes brownish yellow, with a greasy luster, complete cleavage, Mohs hardness of 5-5.5, and specific gravity of 4.9-5.5.
The main ore mineral of some placer type rare earth deposits in China is monazite, mainly located in Tongcheng, Hubei, Yueyang, Hunan, Shangrao, Jiangxi, Menghai, Yunnan, and He County, Guangxi. However, the extraction of placer type rare earth resources often does not have economic significance. Solitary stones often contain reflexive thorium elements and are also the main source of commercial plutonium.
3、 Overview of rare earth application in nuclear fusion and nuclear fission based on patent panoramic analysis
After the keywords of rare earth search elements are fully expanded, they are combined with the expansion keys and classification numbers of nuclear fission and nuclear fusion, and searched in the Incopt database. The search date is August 24, 2020. 4837 patents were obtained after simple family merger, and 4673 patents were determined after artificial noise reduction.
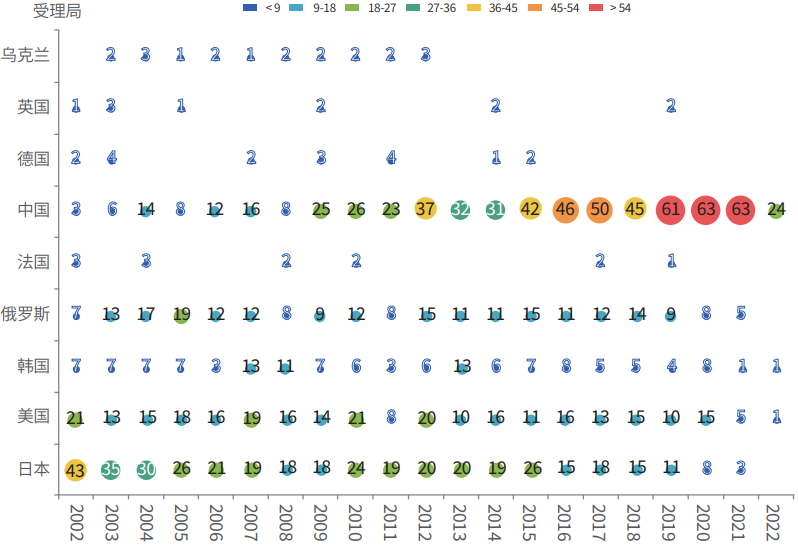
Rare earth patent applications in the field of nuclear fission or nuclear fusion are distributed in 56 countries/regions , mainly concentrated in Japan, China, the United States, Germany and Russia, etc. A considerable number of patents are applied in the form of PCT, of which Chinese patent technology applications have been increasing, especially since 2009, entering a rapid growth stage, and Japan, the United States and Russia have continued to layout in this field for many years (Figure 1).
Figure 1 Application trend of technology patents related to rare earth application in nuclear nuclear fission and nuclear fusion in countries/regions
It can be seen from the analysis of technical themes that the application of rare earth in nuclear fusion and nuclear fission focuses on fuel elements, scintillators, radiation detectors, actinides, plasmas, nuclear reactors, shielding materials, neutron absorption and other technical directions.
4、 Specific Applications and Key Patent Research of Rare Earth Elements in Nuclear Materials
Among them, nuclear fusion and nuclear fission reactions in nuclear materials are intense, and the requirements for materials are strict. At present, power reactors are mainly nuclear fission reactors, and fusion reactors may be popularized on a large scale after 50 years. The application of rare earth elements in reactor structural materials; In specific nuclear chemical fields, rare earth elements are mainly used in control rods; In addition, scandium has also been used in radiochemistry and nuclear industry.
(1) As combustible poison or control rod to adjust neutron level and critical state of nuclear reactor
In power reactors, the initial residual reactivity of new cores is generally relatively high. Especially in the early stages of the first refueling cycle, when all nuclear fuel in the core is new, the remaining reactivity is the highest. At this point, relying solely on increasing control rods to compensate for residual reactivity would introduce more control rods. Each control rod (or rod bundle) corresponds to the introduction of a complex driving mechanism. On the one hand, this increases costs, and on the other hand, opening holes in the pressure vessel head can lead to a decrease in structural strength. Not only is it uneconomical, but it is also not allowed to have a certain amount of porosity and structural strength on the pressure vessel head. However, without increasing the control rods, it is necessary to increase the concentration of chemical compensating toxins (such as boric acid) to compensate for the remaining reactivity. In this case, it is easy for the boron concentration to exceed the threshold, and the temperature coefficient of the moderator will become positive.
To avoid the aforementioned problems, a combination of combustible toxins, control rods, and chemical compensation control can generally be used for control.
(2) As a dopant to enhance the performance of reactor structural materials
Reactors require structural components and fuel elements to have a certain level of strength, corrosion resistance, and high thermal stability, while also preventing fission products from entering the coolant.
1) .Rare earth steel
The nuclear reactor has extreme physical and chemical conditions, and each component of the reactor also has high requirements for the special steel used. Rare earth elements have special modification effects on steel, mainly including purification, metamorphism, microalloying, and improvement of corrosion resistance. Rare earth containing steels are also widely used in nuclear reactors.
① Purification effect: Existing research has shown that rare earths have a good purification effect on molten steel at high temperatures. This is because rare earths can react with harmful elements such as oxygen and sulfur in the molten steel to generate high-temperature compounds. The high-temperature compounds can be precipitated and discharged in the form of inclusions before the molten steel condenses, thereby reducing the impurity content in the molten steel.
② Metamorphism: on the other hand, the oxides, sulfides or oxysulfides generated by the reaction of rare earth in the molten steel with harmful elements such as oxygen and sulfur can be partially retained in the molten steel and become inclusions of steel with high melting point. These inclusions can be used as heterogeneous nucleation centers during solidification of the molten steel, thus improving the shape and structure of steel.
③ Microalloying: if the addition of rare earth is further increased, the remaining rare earth will be dissolved in the steel after the above purification and metamorphism are completed. Since the atomic radius of rare earth is larger than that of iron atom, rare earth has higher surface activity. During the solidification process of molten steel, rare earth elements are enriched at the grain boundary, which can better reduce the segregation of impurity elements at the grain boundary, thus strengthening the solid solution and playing the role of microalloying. On the other hand, due to the hydrogen storage characteristics of rare earths, they can absorb hydrogen in steel, thereby effectively improving the hydrogen embrittlement phenomenon of steel.
④ Improving corrosion resistance: The addition of rare earth elements can also improve the corrosion resistance of steel. This is because rare earths have a higher self corrosion potential than stainless steel. Therefore, the addition of rare earths can increase the self corrosion potential of stainless steel, thereby improving the stability of steel in corrosive media.
2). Key Patent Study
Key patent: invention patent of an oxide dispersion strengthened low activation steel and its preparation method by Institute of Metals, Chinese Academy of Sciences
Patent abstract: Provided is an oxide dispersion strengthened low activation steel suitable for fusion reactors and its preparation method, characterized in that the percentage of alloy elements in the total mass of the low activation steel is: the matrix is Fe, 0.08% ≤ C ≤ 0.15%, 8.0% ≤ Cr ≤ 10.0%, 1.1% ≤ W ≤ 1.55%, 0.1% ≤ V ≤ 0.3%, 0.03% ≤ Ta ≤ 0.2%, 0.1 ≤ Mn ≤ 0.6%, and 0.05% ≤ Y2O3 ≤ 0.5%.
Manufacturing process: Fe-Cr-W-V-Ta-Mn mother alloy smelting, powder atomization, high-energy ball milling of the mother alloy and Y2O3 nanoparticle mixed powder, powder enveloping extraction, solidification molding, hot rolling, and heat treatment.
Rare earth addition method: Add nanoscale Y2O3 particles to the parent alloy atomized powder for high-energy ball milling, with the ball milling medium being Φ 6 and Φ 10 mixed hard steel balls, with a ball milling atmosphere of 99.99% argon gas, a ball material mass ratio of (8-10): 1, a ball milling time of 40-70 hours, and a rotational speed of 350-500 r/min.
3).Used to make neutron radiation protection materials
① Principle of neutron radiation protection
Neutrons are components of atomic nuclei, with a static mass of 1.675 × 10-27kg, which is 1838 times the electronic mass. Its radius is approximately 0.8 × 10-15m, similar in size to a proton, similar to γ Rays are equally uncharged. When neutrons interact with matter, they mainly interact with the nuclear forces inside the nucleus, and do not interact with the electrons in the outer shell.
With the rapid development of nuclear energy and nuclear reactor technology, more and more attention has been paid to nuclear radiation safety and nuclear radiation protection. In order to strengthen radiation protection for operators who have been engaged in radiation equipment maintenance and accident rescue for a long time, it is of great scientific significance and economic value to develop lightweight shielding composites for protective clothing. Neutron radiation is the most important part of nuclear reactor radiation. Generally, most of the neutrons in direct contact with human beings have been slowed down to low-energy neutrons after the neutron shielding effect of the structural materials inside the nuclear reactor. Low energy neutrons will collide with nuclei with lower atomic number elastically and continue to be moderated. The moderated thermal neutrons will be absorbed by elements with larger neutron absorption cross sections, and finally neutron shielding will be achieved.
② Key Patent Study
The porous and organic-inorganic hybrid properties of rare earth element gadolinium based metal organic skeleton materials increase their compatibility with polyethylene, promoting the synthesized composite materials to have higher gadolinium content and gadolinium dispersion. The high gadolinium content and dispersion will directly affect the neutron shielding performance of the composite materials.
Key patent: Hefei Institute of Material Science, Chinese Academy of Sciences, invention patent of a gadolinium based organic framework composite shielding material and its preparation method
Patent Abstract: Gadolinium based metal organic skeleton composite shielding material is a composite material formed by mixing gadolinium based metal organic skeleton material with polyethylene in a weight ratio of 2:1:10 and forming it through solvent evaporation or hot pressing. Gadolinium based metal organic skeleton composite shielding materials have high thermal stability and thermal neutron shielding ability.
Manufacturing process: selecting different gadolinium metal salts and organic ligands to prepare and synthesize different types of gadolinium based metal organic skeleton materials, washing them with small molecules of methanol, ethanol, or water by centrifugation, and activating them at high temperature under vacuum conditions to fully remove the residual unreacted raw materials in the pores of the gadolinium based metal organic skeleton materials; The gadolinium based organometallic skeleton material prepared in step is stirred with polyethylene lotion at a high speed, or ultrasonically, or the gadolinium based organometallic skeleton material prepared in step is melt blended with ultra-high molecular weight polyethylene at high temperature until fully mixed; Place the uniformly mixed gadolinium based metal organic skeleton material/polyethylene mixture in the mold, and obtain the formed gadolinium based metal organic skeleton composite shielding material by drying to promote solvent evaporation or hot pressing; The prepared gadolinium based metal organic skeleton composite shielding material has significantly improved heat resistance, mechanical properties, and superior thermal neutron shielding ability compared to pure polyethylene materials.
Rare earth addition mode: Gd2 (BHC) (H2O) 6, Gd (BTC) (H2O) 4 or Gd (BDC) 1.5 (H2O) 2 porous crystalline coordination polymer containing gadolinium, which is obtained by coordination polymerization of Gd (NO3) 3 • 6H2O or GdCl3 • 6H2O and organic carboxylate ligand; The size of gadolinium based metal organic skeleton material is 50nm-2 μ m; Gadolinium based metal organic skeleton materials have different morphologies, including granular, rod-shaped, or needle shaped shapes.
(4) Application of Scandium in Radiochemistry and nuclear industry
Scandium metal has good thermal stability and strong fluorine absorption performance, making it an indispensable material in the atomic energy industry.
Key patent: China Aerospace Development Beijing Institute of Aeronautical Materials, invention patent for an aluminum zinc magnesium scandium alloy and its preparation method
Patent abstract: An aluminum zinc magnesium scandium alloy and its preparation method. The chemical composition and weight percentage of the aluminum zinc magnesium scandium alloy are: Mg 1.0% -2.4%, Zn 3.5% -5.5%, Sc 0.04% -0.50%, Zr 0.04% -0.35%, impurities Cu ≤ 0.2%, Si ≤ 0.35%, Fe ≤ 0.4%, other impurities single ≤ 0.05%, other impurities total ≤ 0.15%, and the remaining amount is Al. The microstructure of this aluminum zinc magnesium scandium alloy material is uniform and its performance is stable, with an ultimate tensile strength of over 400MPa, a yield strength of over 350MPa, and a tensile strength of over 370MPa for welded joints. The material products can be used as structural elements in aerospace, nuclear industry, transportation, sporting goods, weapons and other fields.
Manufacturing process: Step 1, ingredient according to the above alloy composition; Step 2: Melt in the smelting furnace at a temperature of 700 ℃~780 ℃; Step 3: Refine the completely melted metal liquid, and maintain the metal temperature within the range of 700 ℃~750 ℃ during refining; Step 4: After refining, it should be fully allowed to stand still; Step 5: After fully standing, start casting, maintain the furnace temperature within the range of 690 ℃~730 ℃, and the casting speed is 15-200mm/minute; Step 6: Perform homogenization annealing treatment on the alloy ingot in the heating furnace, with a homogenization temperature of 400 ℃~470 ℃; Step 7: Peel the homogenized ingot and perform hot extrusion to produce profiles with a wall thickness of over 2.0mm. During the extrusion process, the billet should be maintained at a temperature of 350 ℃ to 410 ℃; Step 8: Squeeze the profile for solution quenching treatment, with a solution temperature of 460-480 ℃; Step 9: After 72 hours of solid solution quenching, manually force aging. The manual force aging system is: 90~110 ℃/24 hours+170~180 ℃/5 hours, or 90~110 ℃/24 hours+145~155 ℃/10 hours.
5、 Research Summary
On the whole, rare earths are widely used in nuclear fusion and nuclear fission, and have many patent layouts in such technical directions as X-ray excitation, plasma formation, light water reactor, transuranium, uranyl and oxide powder. As for reactor materials, rare earths can be used as reactor structural materials and related ceramic insulation materials, control materials and neutron radiation protection materials.
Post time: May-26-2023