Lutetium is a rare rare earth element with high prices, minimal reserves, and limited uses. It is soft and soluble in dilute acids, and can slowly react with water.
The naturally occurring isotopes include 175Lu and a half-life of 2.1 × 10 ^ 10 years old β Emitter 176Lu. It is made by reducing Lutetium(III) fluoride LuF ∨ · 2H ₂ O with calcium.
The main use is as a catalyst for petroleum cracking, alkylation, hydrogenation, and polymerization reactions; In addition, Lutetium tantalate can also be used as the material of X-ray fluorescent powder; 177Lu, a radionuclide, can be used for radiotherapy of tumors.

Discovering History
Discovered by: G. Urban
Discovered in 1907
Lutetium was separated from ytterbium by French chemist Ulban in 1907 and was also a rare earth element discovered and confirmed in the early 20th century. The Latin name for lutetium comes from the ancient name of Paris, France, which is the birthplace of Urban. The discovery of lutetium and another rare earth element europium completed the discovery of all rare earth elements present in nature. Their discovery can be considered as opening the fourth gate to the discovery of rare earth elements and completing the fourth stage of rare earth element discovery.
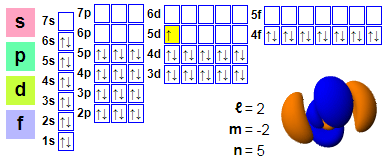
Electron configuration
Electronic arrangements:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d1
Lutetium is a silver white metal, which is the hardest and densest metal among rare earth elements; Melting point 1663 ℃, boiling point 3395 ℃, density 9.8404. Lutetium is relatively stable in the air; Lutetium oxide is a colorless crystal that dissolves in acids to form corresponding colorless salts.
The rare earth metallic luster of lutetium is between silver and iron. The impurity content has a significant impact on their properties, so there are often significant differences in their physical properties in literature.
Metal yttrium, gadolinium, and lutetium have strong corrosion resistance and can maintain their metallic luster for a long time
Application
Due to production difficulties and high prices, lutetium has few commercial uses. The properties of lutetium are not significantly different from other lanthanide metals, but its reserves are relatively smaller, so in many places, other lanthanide metals are usually used to replace lutetium.
Lutetium can be used to make some special alloys, such as Lutetium aluminum alloy can be used for Neutron activation analysis. Lutetium can also be used as a catalyst for petroleum cracking, alkylation, hydrogenation, and polymerization reactions. In addition, doping lutetium in some laser crystals such as Yttrium aluminium garnet can improve its laser performance and optical uniformity. In addition, lutetium can also be used for phosphors: Lutetium tantalate is the most compact white material known at present, and is an ideal material for X-ray phosphors.
177Lu is a synthetic radionuclide, which can be used for radiotherapy of tumors.
Lutetium oxide doped cerium yttrium lutetium silicate crystal
Post time: Jun-26-2023