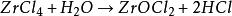Zirconium (IV) chloride, also known as zirconium tetrachloride, has the molecular formula ZrCl4 and a molecular weight of 233.04. Mainly used as analytical reagents, organic synthesis catalysts, waterproofing agents, tanning agents
Product name:Zirconium chloride;Zirconium tetrachloride; Zirconium(IV) chloride
Molecular weight:233.04
EINECS :233-058-2
Boiling point:331 (sublimation)
Density:2.8
Chemical formula:ZrCl4
CAS :10026-11-6
Melting point:437
Water solubility:Soluble in cold water
1.Properties
Physical and chemical properties
1. Character: White glossy crystal or powder, easily deliquescent.
2. Melting point (℃): 437 (2533.3kPa)
3. Boiling point (℃): 331 (sublimation)
4. Relative density (water=1): 2.80
5. Saturated vapor pressure (kPa): 0.13 (190 ℃)
6. Critical pressure (MPa): 5.77
7. Solubility: Soluble in cold water, ethanol, and ether, insoluble in benzene, carbon tetrachloride, and carbon disulfide.
Easy to absorb moisture and moisture, hydrolyzed into hydrogen chloride and zirconium oxychloride in humid air or aqueous solution, the equation is as follows:
Stability
1. Stability: Stable
2. Prohibited substances: water, amines, alcohols, acids, esters, ketones
3. Conditions to avoid contact: humid air
4. Polymerization hazard: non polymerization
5. Decomposition product: chloride
2.Aplication
(1) Used for making metal zirconium, pigments, textile waterproofing agents, leather tanning agents, etc.
(2) Used for the production of zirconium compounds and organic metal organic compounds, it can be used as a solvent and a purifying agent for remelted magnesium metal, with the effects of removing iron and silicon.
3.Synthetic method
Weigh zirconia and calcined carbon black according to the molar ratio of measurement, mix evenly and place them in a porcelain boat. Place the porcelain boat in a porcelain tube and heat it to 500 ℃ in a chlorine gas stream for calcination. Collect the product using a trap at room temperature. Considering the sublimation of zirconium tetrachloride at 331 ℃, a 600mm long tube can be used to re sublimate it in a hydrogen gas stream at 300-350 ℃ to remove oxides and ferric chloride in zirconium chloride.
4. Impact on the environment
Health hazards
Invasion route: inhalation, ingestion, skin contact.
Health hazard: Inhalation can cause respiratory irritation, do not swallow. It has strong irritation and can cause skin burns and eye damage. Oral administration may cause burning sensation in the mouth and throat, nausea, vomiting, watery stools, bloody stools, collapse, and convulsions.
Chronic effects: Causes skin granuloma. Mild irritation to the respiratory tract.
Toxicology and Environment
Acute toxicity: LD501688mg/kg (oral administration to rats); 665mg/kg (mouse oral)
Hazardous characteristics: When subjected to heat or water, it decomposes and releases heat, releasing toxic and corrosive smoke.
Combustion (decomposition) product: hydrogen chloride.
Laboratory monitoring method: Plasma spectroscopy (NIOSH method 7300)
Determination in air: collect the sample with a filter, dissolve it with acid, and then measure it with atomic absorption spectroscopy.
Environmental standards: Occupational Safety and Health Administration (1974), Air Time Weighted Average 5.
Leakage emergency response
Isolate the leakage contaminated area, set up warning signs around it, and suggest emergency treatment personnel to wear gas mask and chemical protective clothing. Do not come into direct contact with the leaked material, avoid dust, carefully sweep it up, prepare a solution of about 5% water or acid, gradually add dilute ammonia water until precipitation occurs, and then discard it. You can also rinse with a large amount of water, and dilute the washing water into the wastewater system. If there is a large amount of leakage, remove it under the guidance of technical personnel. Waste disposal method: Mix the waste with sodium bicarbonate, spray with ammonia water, and add crushed ice. After the reaction stops, rinse with water into the sewer.
Protective measures
Respiratory protection: When exposed to dust, a gas mask should be worn. Wear a self-contained breathing apparatus when necessary.
Eye protection: Wear chemical safety goggles.
Protective clothing: Wear work clothes (made of anti-corrosion materials).
Hand protection: Wear rubber gloves.
Other: After work, take a shower and change clothes. Store clothes contaminated with toxins separately and reuse them after washing. Maintain good hygiene habits.
First aid measures
Skin contact: Immediately rinse with water for at least 15 minutes. If there is a burn, seek medical treatment.
Eye contact: Immediately lift the eyelids and rinse with flowing water or physiological saline for at least 15 minutes.
Inhalation: Quickly remove from the scene to a place with fresh air. Maintain unobstructed respiratory tract. Perform artificial respiration if necessary. Seek medical attention.
Ingestion: When the patient is awake, rinse their mouth immediately, do not induce vomiting, and drink milk or egg white. Seek medical attention.
Fire extinguishing method: foam, carbon dioxide, sand, dry powder.
5.Storage method
Store in a cool, dry, and well ventilated warehouse. Keep away from sparks and heat sources. The packaging must be sealed and protected from moisture. It should be stored separately from acids, amines, alcohols, esters, etc., and avoid mixing storage. The storage area should be equipped with suitable materials to contain leaks.
6 computational chemistry Data Editing
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond receptors: 0
4. Number of rotatable chemical bond: 0
5. Number of tautomers: None
6. Topological molecule polarity surface area: 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 19.1
10. Number of Isotope Atoms: 0
11. Determine the number of atomic structure centers: 0
12. Number of uncertain atomic construction centers: 0
13. Determine the number of chemical bond stereo centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Post time: May-25-2023